25
2023
-
09
Submission requirements for in vitro diagnostic reagent declaration data sheets/labels
Classification:
Industry News
The state continues to strengthen the registration management of medical devices, and the requirements for the materials submitted by our applicants are "complete materials and meet the statutory form requirements". Today, let's talk about the submission requirements of the instructions/label materials in the in vitro diagnostic reagent application materials.
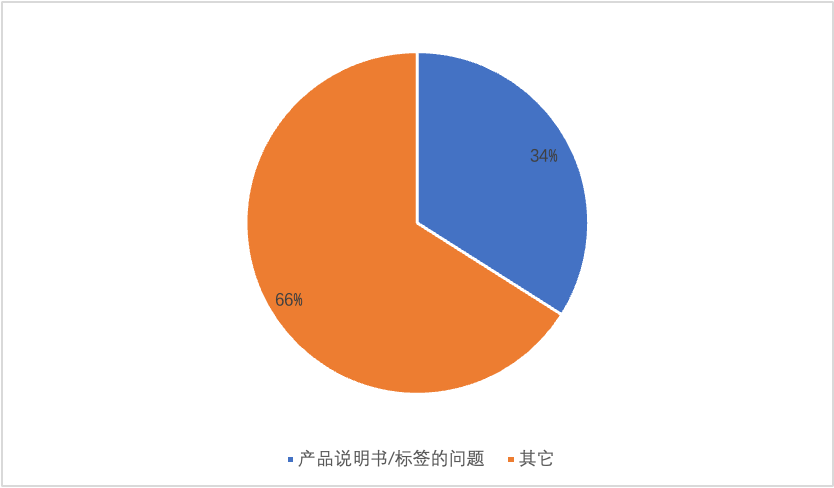
According to the 2019-2021 period published by Fujian Province, in the statistics of defective items for the first registration and application data of the second type of in vitro diagnostic reagent products, the problem of product specifications/labels accounted for 34%, occupying the first place in the statistics of defective items.
1. In vitro diagnostic reagent instructions:
In September 2021, the State Drug Administration issued the "Announcement on the Publication of In Vitro Diagnostic Reagent Registration Application Data Requirements and Approval of the Format of Proof Documents" in Annex 4, "In Vitro Diagnostic Reagent Registration Application Data Requirements and Instructions", the requirements for product specifications and labeling samples are as follows:

According to the requirements, the product instructions of in vitro diagnostic reagents need to conform to the "Guidelines for Writing Instructions for In Vitro Diagnostic Reagents". The current guiding principle is the 2014 edition. In this guiding principle, the format of the product instructions for in vitro diagnostic reagents and the writing of various contents are explained in detail, which provides principled guidance and reference for us to write the product instructions for in vitro diagnostic reagents.

Among them, in vitro diagnostic reagent specification format requirements are as follows:
×××× (product common name) manual
[product name]]
[packing specification]]
intended use]
inspection principle]
main component]
Storage conditions and period of validity]
applicable instrument]
sample requirements]
test method]
Positive judgment value or reference interval]
interpretation of test results]
Limitations of test methods]
product performance index]
[Note]]
References]
[Registrant] (or [Filing Person])
[Medical Device Registration Certificate No.] (or [Medical Device Filing Certificate No.])
production enterprise]
[Medical Device Manufacturer License No.] (or [Medical Device Production Filing Certificate No.])
[date of approval and modification of specification]]
2. Label:
The content of the label sample shall comply with the Regulations on the Administration of Medical Device Instructions and Labels. The current version is issued by the State Food and Drug Administration in July 2014:

Article 13 of the "Regulations on the Administration of Medical Device Instructions and Labels" specifies in detail the general contents to be included in the label, and mentions at the end: "If the medical device label cannot fully indicate the above contents due to limited location or size, at least the product name, model, specification, production date and service life or expiration date shall be marked, and it shall be clearly stated in the label that" see the manual for other contents '."
At the same time, in Article 14 of the Regulations on the Administration of Medical Device Instructions and Labels, the contents that are not allowed in the product instructions and labels are explained in detail:

Previous article
Related News
Address: No.1 Factory Building, No.4, Industrial South Road, Licheng District, Jinan City, Shandong Province

Mobile site


